Containment
Call for experts
World Health Organization is seeking experts to serve as members of the Containment Working Group (CWG) of the Global Commission for the Certification of the Eradication of Poliomyelitis (GCC). Please see the documents below for details on how to apply. Applications close 15 April 2024.
What is containment?
Containment includes biosafety and biosecurity requirements for laboratories, vaccine production sites, or any other facility that handles or stores eradicated polioviruses, to minimize the risk of these viruses being released into the community. Containment also concerns risk mitigation measures associated with field use of some live oral polio vaccines. Containment is a key objective of the GPEI’s Polio Eradication Strategy 2022-2026, and will be critical for achieving and maintaining a polio-free world.
Two of three strains of wild poliovirus have been declared globally eradicated. In September 2015, the Global Commission for the Certification of Eradication of Poliomyelitis declared wild poliovirus type 2 as eradicated, and in October 2019, wild poliovirus type 3 followed. A number of facilities worldwide, however, still handle or store the viruses for activities such as vaccine production, polio diagnostics and research. Further, type 2 or type 3-containing oral polio vaccines, made with weakened, live vaccine virus, continue to be used across the world for outbreak response or routine immunization.
It is important that all poliovirus type 2, and all wild and vaccine-derived poliovirus (VDPV) type 3, is destroyed, or safely and securely contained so that these viruses are not released from facilities that retain them. It is also important that inventories and appropriate destruction of unused vaccine containing live type 2 strains is conducted as per GPEI outbreak response guidelines. Currently, measures only apply to live type 2-containing vaccines used for outbreak response.
The accidental or deliberate release of the virus from one of these facilities, or the potential emergence of VDPV due to vaccine mismanagement in areas of low population immunity could result in the return of these strains.
Containment activities
At the 71st World Health Assembly, Member States adopted a resolution which urges the intensification of efforts to accelerate progress towards poliovirus containment globally.
Activities are underway to ensure that all poliovirus type 2, and type 3 wild and vaccine-derived materials are either destroyed, or safely and securely contained. This involves conducting national inventories of facilities that handle or store these materials, and ensuring appropriate destruction of unneeded virus. Governments that have designated facilities for the retention of virus, for what they deem are critical functions, are required to ensure certification of these facilities against WHO poliovirus containment guidance.
Similar activities for facilities that handle or store OPV2 and Sabin2 materials are in progress.
Minimizing the number of government designated ‘poliovirus–essential’ facilities reduces the risk of release of the virus and allows for stronger national and international oversight of containment activities, strengthening the likelihood that global containment standards can be met and maintained. Additionally, ensuring high quality response to VDPV2 outbreaks reduces the need for deployment of live type 2-containing vaccines.
Watch our animated video on containment.
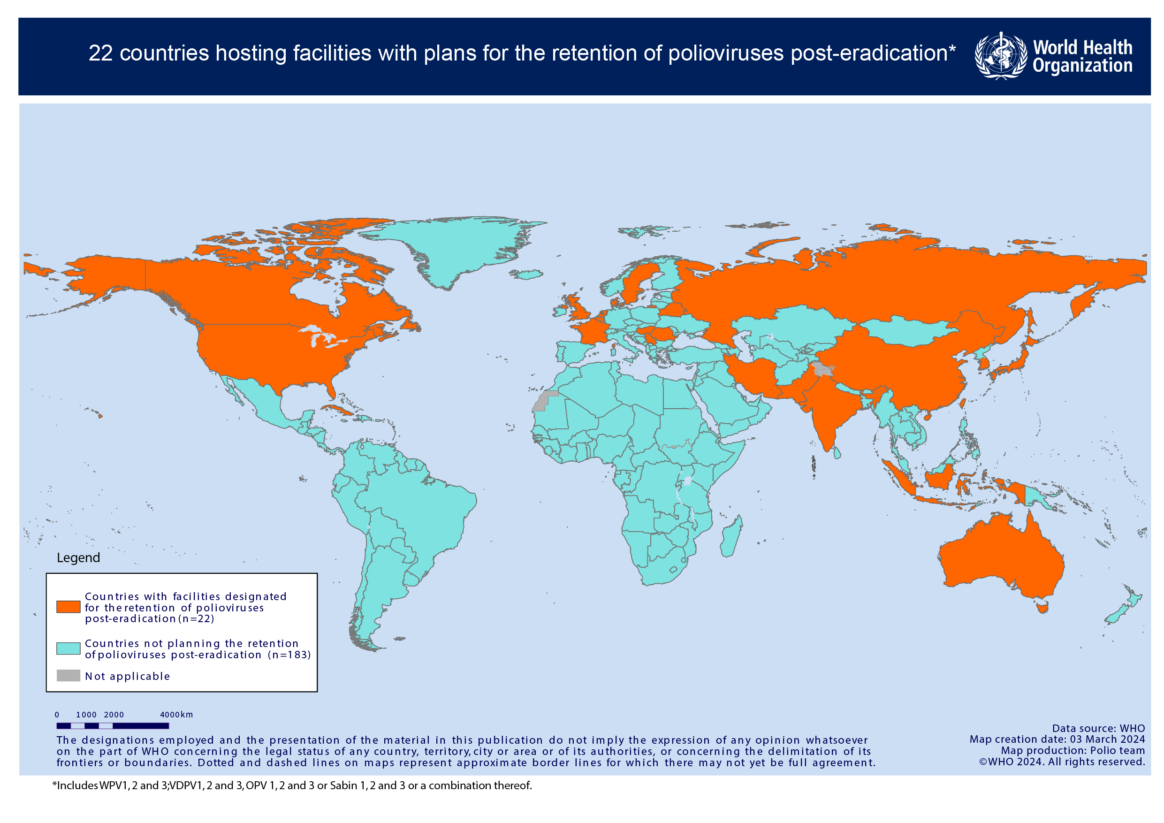
Click here to view country progress towards poliovirus containment certification.







