Vaccine Vial Monitors – helping save lives!
Time temperature indicator known as a vaccine vial monitor (VVM) absolutely vital to eradication effort; allowing health workers to know vaccine has not been exposed to excessive heat.

Have you ever noticed the purple circle on every vial of oral polio vaccine (OPV)? No, it is not a logo! In fact, it is a time temperature indicator known as a vaccine vial monitor (VVM), and it is absolutely vital to the eradication effort.
OPV must be maintained at controlled temperatures to remain effective. But often, vaccination campaigns are conducted in extremely hot weather conditions. So how can it be ensured that the vaccine being delivered to children has not been exposed to heat that could cause damage? Well, that’s where the VVM comes in!
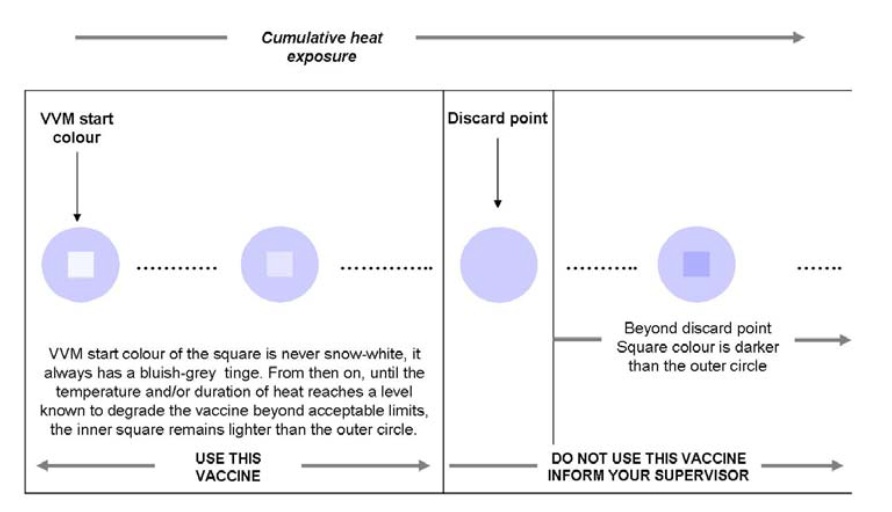
VVMs ingeniously change colour when the vaccine is exposed to heat over time. And so this little unassuming label affixed on the side or the cap of each container of OPV in fact allows health workers to know that the vaccine has not been exposed to excessive heat (as shown in the diagram below).
But how did it come about that VVMs are now on every single OPV vial distributed through the Global Polio Eradication Initiative (GPEI)?
In the 1990s, there was grave concern that children vaccinated against serious diseases, such as polio, might not be protected if the vaccine lost its efficacy due to heat damage. The World Health Organization (WHO), in collaboration with PATH, challenged industry to develop an accurate, easy-to-use and cost-effective device that could monitor heat exposure of vaccines over time.
“We responded to WHO’s challenge,” said Renaat Van den Hooff, President of Temptime Corporation, which manufactures VVMs. “We set out to produce accurate, reliable VVMs, which can be easily affixed to each container, are reliable in field conditions and which can be produced in large volumes necessary for the GPEI.”
As a result, since 1996, billions of VVMs have been affixed to vaccines across the world, and not just for OPV. VVMs are used to ensure that hepatitis B, diphtheria, measles, mumps, pertussis, rabies, rotavirus, rubella, tetanus, tuberculosis and yellow fever vaccines have not been exposed to levels of heat possibly to have damaged them.
In 2005 and again in 2009, Temptime again responded to a critical challenge of the Global Polio Eradication Initiative, when the new monovalent OPVs and bivalent OPVs were introduced to the global eradication effort. Temptime ensured a rapid calibration of its VVMs for these new vaccines, allowing a roll-out of these important new tools in record time. In total, Temptime now produces more than 155 million VVMs for various OPV formulations and an additional 350 million for all other vaccines every single year – a formidable achievement for a company with just 63 employees.
“Vaccines produced by more than 20 of the world’s largest manufacturers have added the protection of a VVM on each vial of life-saving vaccine,” Van den Hooff continued.
Saving lives in a cost-effective way
Critically, VVMs have been shown to save lives in a cost-effective way. A joint study by WHO and PATH from 2006 identified more than 23 million doses of vaccine that had been overexposed to heat, and therefore were not administered to children. This is critical, as it clearly identified that these children could then be targeted for a future vaccine intervention, to ensure they were administered with an effective vaccine, as opposed to being marked in data as having received vaccine (when in fact, the vaccine could have been ineffective). In this way, VVMs help ascertain a clearer picture of the true vaccination coverage in a given population.
The same study identified 31 million doses that had been exposed to potentially-damaging heat, but were still useable – thus greatly avoiding vaccine wastage. WHO estimates that over the next ten years, VVMs will enable the delivery of an additional 140 million doses of vaccine, saving 140,000 lives and decreasing the morbidity rates for countless others.
Related information:
- ‘Five senses’: VVMs and handling vaccines with great care! This video shows the challenges of delivering vaccines from the manufacturing facility to remote areas.