Summer 2010, no.6
Among other topics, in this issue you will find overviews of the programmatic benefits of bivalent OPV, identifying and filling epidemiological gaps, the importance of optimizing outbreak response, pre-empting outbreaks
Articles
In 2008, alarmed that polio remained entrenched in the four countries which had never stopped transmission of wild poliovirus, the World Health Assembly (WHA) called for the development of a new strategy to complete polio eradication. Since then, the Global Polio Eradication Initiative (GPEI) introduced a major programme of work to help develop new approaches to interrupt the remaining chains of wild poliovirus transmission. Underpinning the finalization of this new Strategic Plan has been an accelerated research agenda to develop and evaluate new tools and approaches that directly tackle the remaining barriers to eradication in the lingering polio endemic areas, that facilitate a swifter, more thorough outbreak response, and that limits renewed international spread of polio.
The accelerated research agenda has focused on a vast array of cross-cutting and country-specific studies. The game-changing bivalent oral polio vaccine (OPV) was evaluated and developed in record time; the Short Interval Additional Dose (SIAD) strategy was introduced in conflict-affected and outbreak settings; social research was conducted to tailor social mobilization strategies in India, Nigeria and Pakistan; trials were held to more clearly assess vaccine efficacy in multiple settings; seroconversion studies focused on validating supplementary immunization activity (SIA) performance in key reservoir areas; and, mathematical modelling and case-control studies were employed to more clearly highlight areas at particular risk of outbreaks following re-infection. New methods to monitor SIA operations were pioneered to help guide mid-course corrections and new strategies were examined to further boost the efficacy of OPVs and close susceptibility gaps in targeted communities. Finally, supplemental surveillance strategies – including expanding environmental surveillance to key urban reservoir areas – were explored.
All of these new approaches, the implementation of which has already had a significant epidemiological impact, particularly in the traditional reservoir areas of northern India and northern Nigeria, have now been institutionalized in the published GPEI Strategic Plan 2010-2012, which was endorsed by the WHA in May. Mr Bill Gates, co-chair of the Bill and Melinda Gates Foundation, told the United States Congress in March 2010 that this new Plan will “strike at the final reservoirs of polio and consign this terrible virus to history”.
There has never been a better opportunity to achieve a polio-free world, as the GPEI is now armed with the required insight and knowledge to tackle the remaining challenges. Due to the fact research will play a key role in both monitoring the implementation of the new strategy and in further sensitizing the approaches, this issue of Polio Pipeline examines in greater depth the role research has played in developing this new strategy, and how it will contribute to its implementation.
Following the development and wide utilisation of monovalent oral polio vaccines (OPV) since 2005, transmission of indigenous wild poliovirus type 1 (WPV1) and wild poliovirus type 3 (WPV3) has been restricted to geographically limited areas of four endemic countries: Nigeria, India, Pakistan, and Afghanistan.
However, given the ongoing co-circulation of WPV1 and WPV3 in these areas, in November 2007, the Advisory Committee on Poliomyelitis Eradication (ACPE) recommended that the Global Polio Eradication Initiative (GPEI) exploit an opportunity to obtain clinical data on a bivalent OPV (containing type 1 and 3 serotypes) in a clinical trial.
In 2008-2009, the clinical trial was conducted in India (in Indore, Pune and Chennai), to compare the rate of seroconversion to each serotype in the bivalent OPV with that of the respective monovalent OPV and trivalent OPV. For both types 1 and 3 polio, bivalent OPV was found to be at least 35% more effective than trivalent OPV and almost as good as the monovalent OPVs. The ACPE reviewed the final trial results in November 2009 and concluded that the strategic use of bivalent OPV in supplementary immunization activities (SIAs) could be an important additional tool in polio eradication, in those areas where both serotypes are circulating.
Since then, bivalent OPV products from four vaccine manufacturers have been pre-qualified by WHO, while a further two applications for pre-qualification are pending. Between December 2009 and May 2010, over 300 million doses have been procured and utilized in nine countries, with a further rapid scale up of utilization expected throughout 2010 (Table 1). This new vaccine complements the existing arsenal of monovalent and trivalent OPVs, but fills a previously key gap by simultaneously generating immunity to both remaining WPV serotypes. The new bivalent OPV has greatly simplified the logistics of conducting SIAs, and it is anticipated that this tool could greatly accelerate eradication in some settings. The rapid scale-up of the new bivalent OPV is expected to be a cornerstone approach to optimizing SIA strategy during the life of the new GPEI Strategic Plan 2010-2012.
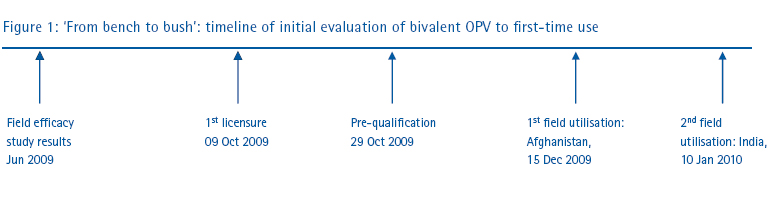
The field evaluation, development and availability of bivalent OPV further reflects the dynamic nature of the GPEI: evidence-based programming, ongoing learning and tactical adjustments to provide the best possible response to field challenges and requirements (Figure 1). The very rapid process from field test to actual field application of this vaccine was the result of an extraordinary collaboration between WHO, UNICEF, vaccine manufacturers and national regulatory agencies.
Table 1 – Utilization of bivalent OPV, by country, during SIAs
| Country | Date of SIA | Number of doses of bivalent OPV used (in millions) |
| Afghanistan | 15 December 2009 | 4.6 |
| 24 January 2010 | 0.5 | |
| 14 February 2010 | 4.6 | |
| 14 March 2010 | 9.4 | |
| Pakistan | 15 February 2010 | 38.5 |
| 15 March 2010 | 19.2 | |
| 24 May 2010 | 19.2 | |
| Sudan | 22 February 2010 | 8 |
| 29 March 2010 | 3.4 | |
| India | 10 January 2010 | 15.6 |
| 7 February 2010 | 40.6 | |
| 25 April 2010 | 44.5 | |
| 23 May 2010 | 39.4 | |
| Nepal | 10 April 2010 | 5.8 |
| 15 May 2010 | 5.8 | |
| Benin | 24 April 2010 | 3.3 |
| Niger | 26 March 2010 | 5.1 |
| 24 April 2010 | 5.1 | |
| Nigeria | 30 January 2010 | 51.8 |
| 24 April 2010 | 18.6 | |
| GRAND TOTAL | 344.4 |
As control activities to achieve eradication become more sophisticated by targeting specific poliovirus serotypes with type-specific monovalent oral polio vaccines (OPV) or with the new bivalent OPV containing type 1 and 3 serotypes (particularly where co-circulation of both serotypes exists), there is a concurrent need to better assess the coverage achieved during supplemental immunization activities (SIAs) and to better measure the impact of the SIAs on actual population immunity against polioviruses.
As a supplement to existing monitoring procedures, and to help assess vaccination coverage achieved during SIAs, the Global Polio Eradication Initiative (GPEI) adopted the existing methodology of Lot Quality Assurance Sampling (LQAS) to the specific needs of the eradication programme. In November 2009, the new cluster LQAS methods were piloted in Nigeria, and applied subsequently over the next six months in Nigeria and other countries in the WHO African Region.
To better measure population immunity, a number of seroprevalence surveys have been conducted or are planned for the next six months, both in Asia (ie India and Pakistan) and Africa (ie Nigeria). These seroprevalence surveys usually focus on very young infants, ages 6-11 months, to provide the most conservative population immunity profiles (immunity in these young infants is lowest since maternally-derived antibodies have waned and they have received relatively fewer doses of polio vaccines than older age groups). At end-2009, the GPEI had already documented a marked increase in type 1 immunity in northern India, from ~80% in 2007 to >99% in 2009, confirming and validating the corresponding decrease in type 1 poliomyelitis cases in this area.
While both methods provide important data to validate programmatic efforts to refocus the vaccine mix if necessary, or to provide confidence to programme managers that activities are on the right path, these methods are time- and resource intensive, and should only be used to answer specific important questions. Thus, while both methods are unlikely to become standard tools for the GPEI, these methods will be applied in areas where there are questions about programme performance or where case reporting data may conflict with SIAs coverage data.
Reinfection of previously polio-free countries and the resulting outbreak response activities cost the Global Polio Eradication Initiative (GPEI) close to US$1 billion during 2003-2009. Those countries most at risk of an outbreak have historically been close to remaining endemic areas or had significant gaps in immunization coverage. However, historical patterns are not always a good indicator for future risk. In 2008-2009, polio cases were reported across Africa in a series of outbreaks on an unprecedented scale1. Many of these countries experienced their first polio outbreak after stopping indigenous wild poliovirus transmission.
To understand the factors that put a country at risk of a polio outbreak a team from the World Health Organization (WHO) and Imperial College London studied polio surveillance and demographic information, including movement data from across Africa. Regression models showed that poor routine immunization coverage with three doses of oral polio vaccine (OPV), high levels of exposure to poliovirus from Nigeria through population movement and a young population were all independently associated with a larger number of polio outbreaks. Periodic supplementary immunization activities were implemented in some countries to protect against outbreaks, but were of variable quality over the period of analysis and no strong protective effect was observed.
Country-specific forecasts of outbreak risk six months ahead of time can be produced using routinely reported data on the risk factors that were identified by the regression analysis. These forecasts were found to have a reasonably good predictive ability and identified countries newly at risk in advance of an outbreak, and accurately described the increased number of outbreaks in 2008-2009 and show a reduced risk in 2010 as a result of the recent decline in the number of polio cases reported in Nigeria. Continued use of these forecasts will help optimize resource allocation in Africa to minimize the number of polio outbreaks during 2010-2012.
Resurgence of wild poliovirus types 1 and 3 in 15 African countries, January 2008-March 2009. Weekly Epidemiological Record. No. 16, 2009, 84, 133-140.
Polio vaccines and polio immunization in the pre-eradiation era: WHO position paper. Weekly Epidemiological Record. No. 23, 2010, 85, 213-228.
The supply landscape and economics of IPV-containing combination vaccines: key findings. Commissioned by the Bill and Melinda Gates Foundation. Prepared by Oliver Wyman, May 2010. Available at Global Polio Eradication.
Improving the affordability of inactivated poliovirus vaccines (IPV) for use in low- and middle-income countries – an economic analysis of strategies to reduce the cost of routine IPV immunization. PATH and Working in Tandem Ltd. April 2010. Available at Global Polio Eradication
Senior Programme Officer – New Vaccines Introduction, Bill and Melinda Gates Foundation
Brief job description:
The Global Health Delivery group at the Bill and Melinda Gates Foundation is looking for a Senior Programme Officer (SPO) for its New Vaccines Introduction team. The SPO will be primarily responsible for the development and management of product development and launch strategies to ensure fast, broad, and appropriate access to the technologies being developed by the Bill and Melinda Gates Foundation’s grantees. Initial focus will be on vaccines for pneumonia and diarrheal diseases.
Requirements:
The ideal candidate will bring depth and breadth of experience and knowledge in business strategy, project management skills, and product introduction planning for new health technologies, particularly in developing countries. The candidate will have at least 12 years of experience in international public health, global development and/or pharmaceutical and healthcare industries. S/he must either have experience working with global partners such as WHO or GAVI Alliance on global product/vaccine introduction or have strategic planning expertise in marketing and/or product launches. The position is based in Seattle, USA. To view the full job description, please visit www.gatesfoundation.org/jobs.








