Q&A with Michel Zaffran: cVDPV2 and the vaccine available to stop it
GPEI head Michel Zaffran sheds light on the global cVDPV2 situation, the need for type 2-containing OPV, and implications for containment

Q: Outbreaks of circulating Vaccine-Derived Poliovirus type 2 (cVDPV2) are popping up in a lot of countries. How do you explain this? Did the programme know this would happen after the oral polio vaccine ‘switch’?
There have been 47 cVDPV2 outbreaks in 20 countries since the switch in April 2016. Some of these outbreaks are spreading over more than one country. Taking the three years before the switch as a frame of reference, there were 8 cVDPV2 outbreaks in five countries altogether in 2013, 2014 and 2015.
Based on epidemiological modelling studies, we anticipated cVDPV2 outbreaks following the removal of the type 2 component from oral polio vaccine in 2016, via the trivalent to bivalent OPV “switch”. And we anticipated that VDPV cases would outnumber wild poliovirus cases in the endgame. However, what the modelling did not predict was the number and scale of these outbreaks, some of which have proven very difficult to stop.
The reason we are seeing a growing number of cVDPV2 outbreaks, particularly in Africa, is the result of a growing cohort of children without mucosal immunity to type 2 poliovirus, while at the same time the [polio] programme uses monovalent oral polio vaccine type 2 (mOPV2) to respond to existing cVDPV2 outbreaks.
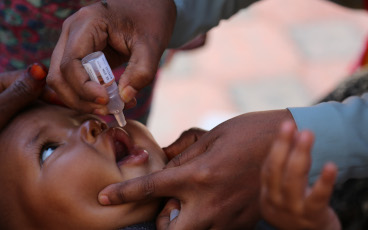
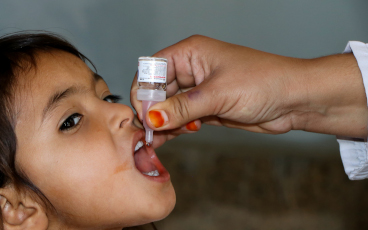
The monovalent vaccine [mOPV2] is currently our only tool to interrupt transmission of cVDPV2 and it is very effective when there is sufficient vaccination coverage in the communities we are targeting to avoid an outbreak. However, when campaign quality is poor and not enough children are reached with the vaccine, we run a risk of seeding new viruses among under-immunized populations. There has been evidence of this happening in and outside of outbreak response zones. We are currently developing a new strategy for stopping cVDPV2 outbreaks, and at the same time preventing new outbreaks.
Q: With a limited global stockpile of mOPV2, is there sufficient vaccine to respond to these and future outbreaks?
No. Current mOPV2 stock is insufficient to cater for the number of outbreaks and the sizes of populations requiring it. The GPEI is working with vaccine manufacturers to boost production of mOPV2 and we expect to meet targeted quantities in 2020.
The vaccine will continue to be used for cVDPV2 outbreak response until a new and more genetically stable oral polio vaccine, known as novel oral polio vaccine type 2 (nOPV2), currently under clinical development, is available.
Q. What does increased production of mOPV2 mean for vaccine manufacturers in terms of containment? On one hand, the polio programme is asking for more live type 2-containing OPV. And on the other, it’s pushing for strict containment of all type 2 wild and Sabin polioviruses.
It’s a balance. The world needs enough mOPV2 stocks to help with the elimination of cVDPV2, and type 2 live attenuated poliovirus is needed to produce this vaccine. Yes, we are asking vaccine manufacturers to make more vaccine, but [vaccine] production and containment of type 2 virus are not mutually exclusive pursuits. Polio vaccine manufacture is costly, particularly when demand calls for rapid scale-up of outputs. Containment is also costly. But this is not a reason to put it on hold and stop efforts to ensure safe and secure handling and storage of virus. Quite the opposite: the impetus for putting in place adequate biorisk management systems should be greater, given the higher level of risk of human exposure to poliovirus in and around these facilities.
Q. What about manufacturers of inactivated polio vaccine (IPV)? Can they afford to relax?
IPV is made with killed, or inactivated strains of wild poliovirus types 1, 2 and 3, or their Sabin counterparts. Any facility manufacturing polio vaccines using the type 2 serotype – be it wild or Sabin ̶ and type 3 wild poliovirus since the declaration of its global eradication in October, is required to implement containment measures set out by WHO. This of course also applies to any other type of facilities holding the viruses, for example, research or diagnostic labs.
Holding on to these viruses is a risk and responsibility, and appropriate measures must be taken to protect communities from reintroduction and resurgence.
The world needs IPV and will continue to need it for the foreseeable future. We need vaccine production to continue in well-managed facilities that incorporate GAPIII approaches to biorisk management.
Q. of Sabin 2 remains a priority, while simultaneously, mOPV2 made up of Sabin 2 is being used in countries around the world. What gives?
First, we must be clear that use of mOPV2 is not a decision that is taken lightly. A thorough risk-benefit analysis is conducted before an advisory committee makes a recommendation and it is submitted to the Director-General of WHO for his approval.
It is never ideal to use mOPV2 and reintroduce Sabin 2, which should be under containment. However, as I mentioned earlier, mOPV2 is currently the only tool available to stop outbreaks of cVDPV2 and we must use it.
The reason we continue to push for containment of Sabin 2 viruses in countries not experiencing cVDPV2 outbreaks is precisely to prevent further emergences of VDPV2, which can cause outbreaks of cVDPV2 more easily now because of the very low population mucosal immunity to type 2 poliovirus.
Q. sounds like we are fighting fire with fire with mOPV2. Are we?
Many outbreaks have been stopped using mOPV2. However, in areas with low routine vaccination coverage, and thus low immunity, we are indeed reintroducing Sabin 2 in naïve populations and seeding new outbreaks. We are currently reviewing all aspects of our cVDPV2 approach and developing a new strategy that examines all options and tools ensuring we are using each for full impact. This includes improving our outbreak response so that it is appropriate in scope and effective, and accelerating the development and roll-out of a new vaccine that is less likely to seed outbreaks.
Q: When will nOPV2 be available?
Clinical trials are underway. There are numerous influencing factors but if all goes according to plan, our estimate is that approximately 100 million doses of the vaccine could be ready by mid-2020, with another 100 million by the end of the year. We are also working with the WHO prequalification team, which independently reviews all vaccine data to ensure a consistent quality in accordance with international standards to enable the vaccine to be used as quickly as possible by affected countries under an Emergency Use Listing (EUL), a risk-based procedure for assessing vaccines for use during public health emergencies—such as polio.
The vaccine is also being developed for types 1 and 3 polioviruses; however, this is further away in terms of production.