OPV
Oral polio vaccine
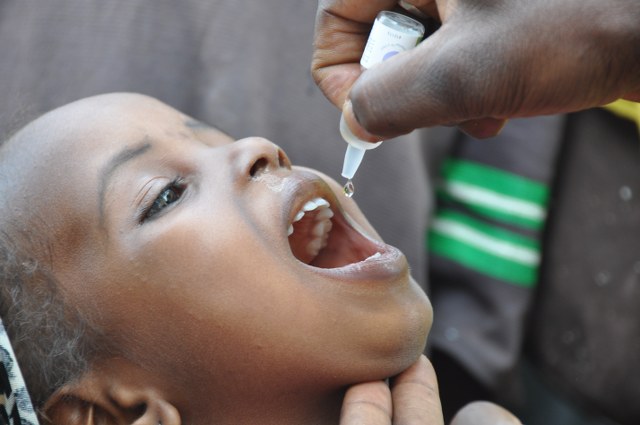
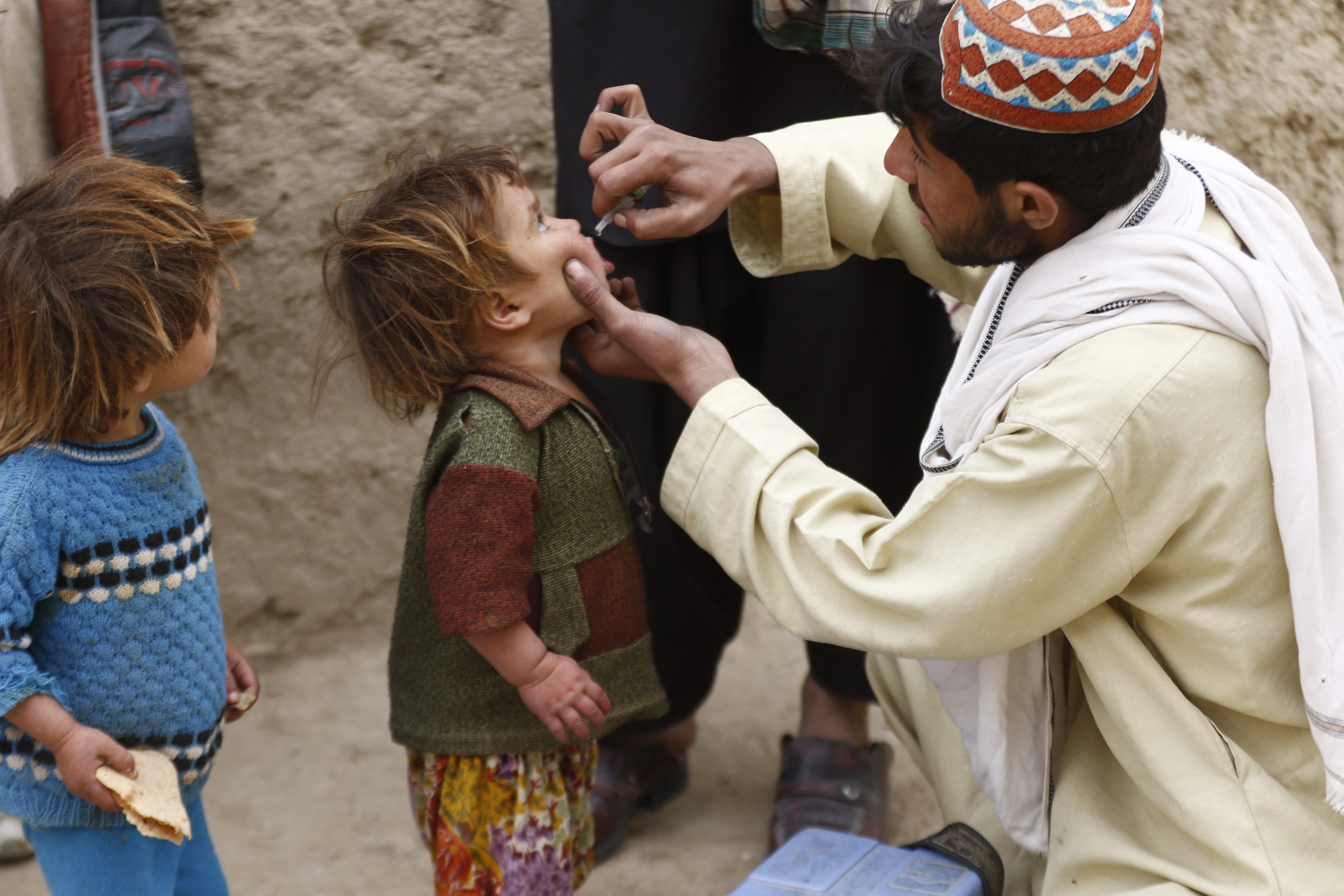
The oral polio vaccine (OPV) is safe and extremely effective, and the most common kind of vaccine used in the fight to eradicate polio. There are different types of OPVs that protect against one, a combination of two, or all three different serotypes of polio – types 1, 2, and/or 3.
Unlike the inactivated polio vaccine (IPV), OPV has a unique ability to stop person-to-person spread of poliovirus. Additionally, it is easier to administer and can be distributed quickly on a large scale. The use of OPV has helped reduce global polio cases by over 99% since 1988, and it remains a vital tool in the effort to end polio everywhere.
Advantages
- OPVs are all inexpensive (US $0.15 – $0.20 for countries procuring through UNICEF).
- OPVs are safe and effective and offer long lasting protection for individuals against the serotype(s) which they target.
- For several weeks after vaccination the vaccine virus replicates in the intestine, is excreted and can be spread to others in close contact. This means that immunization with OPV can result in ‘passive’ immunization of people who have not been vaccinated.
- OPVs are administered orally and do not require health professionals, sterile needle syringes or a complex cold chain system. As such, OPVs are easy to administer in large-scale vaccination campaigns and to transport to hard-to-reach areas.
Disadvantages
- OPV is safe and effective. However, in extremely rare cases (at a rate of approximately 1 in 2.7 million doses) the weakened vaccine-virus in OPV can cause vaccine-associated paralytic poliomyelitis (VAPP). The extremely low risk of VAPP is accepted by public health programmes given the strong protection OPV otherwise provides.
- In places where not enough children are immunized against polio, the weakened vaccine-virus can pass through the community and, in rare instances over time, genetically revert to a form that can cause paralysis. This is known as variant poliovirus, or cVDPV, which is now the most prevalent form of polio.
Bivalent oral polio vaccine (bOPV)
- Bivalent oral polio vaccine (bOPV) is used by many countries around the world in their routine immunization schedules and for response to outbreaks.
- bOPV provides protection against poliovirus types 1 and 3 only. It does not protect against type 2 polio.
- In 2016, bOPV replaced trivalent oral polio vaccine (tOPV) in a globally coordinated vaccine switch, as type 2 wild poliovirus had been eradicated since 1999.
Novel oral polio vaccine type 2 (nOPV2)
- To better address the evolving risk of type 2 variant poliovirus (cVDPV2), the innovative novel oral polio vaccine type 2 (nOPV2) is being deployed in countries affected by these outbreaks.
- nOPV2 is a next-generation version of the monovalent oral polio vaccine type 2 (mOPV2) and provides comparable individual protection against polio while being much more genetically stable and therefore less likely to be associated with new type 2 variant outbreaks.
- Development of nOPV2 began in 2011, and in March 2021, it was rolled out for outbreak response after it was granted WHO Emergency Use Listing (EUL) In December 2023, nOPV2 earned full licensure and WHO Prequalification after rigorous reviews of safety, effectiveness and genetic stability data, as well as quality assurance checks of manufacturing sites.
- nOPV2 is the first EUL-approved vaccine to move through this regulatory pathway, paving the way for other innovations like it. The vaccine is an important tool to help stop variant poliovirus outbreaks more sustainably.
More resources on nOPV2
Trivalent oral poliovirus vaccine (tOPV)
- Prior to April 2016, the trivalent oral poliovirus vaccine (tOPV) was the most common vaccine used for routine immunization against polio.
- tOPV protects against all three serotypes (types 1, 2 and 3).
- The vaccine was withdrawn in April 2016 and replaced with the bivalent oral polio vaccine (bOPV) after type 2 wild poliovirus had been eradicated for close to two decades.
- tOPV remains available for countries to use if more than one type of poliovirus is circulating, i.e. type 2 in addition to type 1 or 3.
Monovalent oral polio vaccine (mOPV)
- Monovalent oral polio vaccines protect against one serotype of polio (type 1, 2, or 3) and are reserved for response to respective outbreaks only.
- Different formulations exist — monovalent OPV for type 1 (mOPV1), for type 2 (mOPV2) and for type 3 (mOPV3).
- mOPV1 and mOPV3 are rarely used, as bOPV is recommended. The program keeps stockpiles of mOPV2 in the event it is needed for response to type 2 variant outbreaks, though nOPV2 continues to be the recommended vaccine (as outlined above).