Autumn 2008, no.2
Polio lab network, new state-of-the-art diagnostic method, the role of mathematical modelling, polio vaccine development: monovalents, seroprevalence surveys.
Articles
A new state-of-the-art diagnostic method – real-time Reverse Transcriptase Polymerase Chain Reaction (rRT-PCR) – has been introduced into the Global Polio Laboratory Network (GPLN). The introduction of rRT-PCR offers several key programmatic advantages to global poliovirus surveillance by:
- permitting rapid and reliable identification of poliovirus isolates
- reducing personnel hands-on time for poliovirus identification
- providing data output in a computerized format
- minimizing the risk of sample contamination that accompany conventional PCR
- leading to development of new screening assays for vaccine-derived polioviruses (VDPVs)
- enabling routine use of PCR reagents capable of detecting specific wild poliovirus genotypes
- setting the stage for direct poliovirus detection by biochemical amplification methods
- opening the way for widespread use of rRT-PCR in surveillance for other vaccine-preventable diseases.
The basic PCR setup consists of two short synthetic single-stranded DNA “primers” that bind to target sequences in an RNA (or DNA) “template”. PCR assays are run as a series of programmed thermal cycles (usually 2–3 minutes/cycle) that are repeated 25–40 times:
- “denaturation” at high temperature (usually 95ºC) to disrupt the base-pairing of the double-stranded template and separate the strands
- “annealing” at lower temperatures (in the range of 44ºC–50ºC) to permit the primers to base-pair with the templates
- “primer extension” at elevated temperatures (in the range of 60ºC– 65ºC), whereby a complementary copy of the template is made by extension of the primer by a thermostable DNA polymerase.
The two primers target complementary strands of the double-stranded DNA template, and at each cycle the number of DNA PCR amplification products (“amplicons”) effectively doubles. The chain lengths of the amplicons are determined by the spacing of the primer binding sites along the templates. Specificities of primer binding are determined by the strength of the base pairs formed between the primers and the nucleic acid templates, and primers can be designed to target any genomic interval. Thus, by a rapid in vitro reaction mimicking natural DNA replication, large amounts of DNA copies can be amplified from minute quantities (as little as one molecule) of original template nucleic acid. When the template is RNA, an initial primer extension step is performed by reverse transcriptase (RT) which makes a DNA copy of the RNA sequence. The basic conventional RT-PCR assay described above has been widely used with high proficiency within the GPLN to identify poliovirus isolates. Primer pairs have been prepared with varying specificities: panEntero (identifies all enteroviruses); panPolio (identifies all polioviruses); SeroPV1, SeroPV2, and SeroPV3 (identifies polioviruses by serotype); and Sab1, Sab2, Sab3 (identifies Sabin strains). Specific amplicons from each primer pair set had different chain lengths, and could easily be separated by gel electrophoresis and visualized.
The original conventional RT-PCR assays to the “real-time” format, whereby the PCR reaction is continuously monitored by special thermal cycling instruments equipped with multichannel fluorescence detectors. In the RT-PCR format, a specific “probe” is included in the reaction, along with the primers. The probe is another short synthetic DNA chain that can form specific stable base pairs with the amplicon. The target sequences on the template for the probe are flanked by the target sequences for the “forward” and “reverse” primers. The probe contains a chemical “fluorophore” (fluoresces when excited by input light) at one (5’) end and a chemical “quencher” (absorbs [“quenches”] the fluorescent light from the fluorophore) at the opposite (3’) end. In rRT-PCR, extension from the primer of the DNA copy along the template causes displacement of the probe from the template and probe degradation. Probe degradation causes the fluorophore and quencher to separate (“uncouple”), so that the fluorescent light emitted by the fluorophore is no longer quenched. At each rRT-PCR cycle, the amount of fluorescent light emitted effectively doubles until the reaction reaches a plateau.
The rRT-PCR format has been field-tested in four GPLN laboratories (CDC-Atlanta, NIH-Islamabad, ERC-Mumbai, NICD Johannesburg) and is poised for wider application within the GPLN.
Transmission of wild poliovirus in the endemic regions of northern Nigeria; Bihar and Uttar Pradesh, India; and cross-border areas of Afghanistan and Pakistan persists simply because children have not been immunized with a sufficient number of doses of oral polio vaccine (OPV) to stop virus circulation.
Although the primary reasons for ongoing vaccination coverage gaps are operational, as indicated by independent monitoring data of supplementary immunization activities (SIAs), increasing community engagement and acceptance of vaccination services can improve coverage and remains a key cornerstone of SIA micro-planning.
Periodic knowledge, attitude and practice (KAP) studies serve as an educational diagnosis of a population or community and are an important way to measure changing beliefs and behaviours over time. Information from KAP studies, when guided by insights from health communication theory, provides formative insights and baseline data. This information helps programmes set communication objectives linked to increased community engagement and demand for vaccination services and develop tailored strategies appropriate for the social, cultural and political contexts of at-risk communities.
Information on where and when caregivers receive information, whether parents perceive polio as a threat to their children or why communities may reject immunization services allow countries to develop messaging and communication strategies to overcome specific programme challenges. When tracked over time, the impact of these strategies towards meeting communication objectives can be measured and evaluated and strategies tailored as necessary. Data from KAP studies and other forms of social research is an important element of the programme that ensures a data-driven, evidence-based approach to communication for polio eradication.
In the last quarter of 2008, KAP studies were planned for Nigeria, Pakistan and India. Afghanistan will conduct a new study in early 2009. Each of the studies will be invaluable to identifying trends and changes in communities, measuring impact of social interventions and to guiding communications planning in 2009 and beyond.
Information from studies conducted in Pakistan and India in 2007 shows the impact of
communication interventions.
For example, in Pakistan, communication activities including interpersonal dialogue and sustained electronic media increased the number of caregivers who saw polio as a threat to 94%, up from 75% in 2005, while 95% of those surveyed now see polio as a health priority for the country compared to only 76% reported in the earlier study. India’s «Underserved Strategy» which focuses on sensitizing minority or underserved populations, especially large Muslim communities in Bihar and Uttar Pradesh, continues to help support the improvement of vaccination coverage and has built ownership and participation from local religious leaders and scholars.
Social research, which includes KAP studies and other activities such as focus groups, community dialogues and in-depth discussions with members of communities, has become a cornerstone for communication planners of polio eradication activities. Similarly, this data has increasingly been applied to help develop tailored eradication tactics to overcome the remaining operational challenges which continue to affect the quality of polio SIAs at country-level.
For those with a special interest in polio communications, “stay tuned” for the results of these important studies in future editions of the Polio pipeline.
Over the past five years, efforts to mathematically model the risks, costs, and benefits of future strategies for managing polio yielded several key insights. Although models cannot predict the future, they provide glimpses of the many possible scenarios and thereby help define the most effective management strategies for the long-term.
Modelling the numerous future policy options related to routine vaccination, supplemental vaccination, surveillance, outbreak response, vaccine stockpile, containment, and other issues clearly demonstrates the need to recognize the differences that exist in national values, preferences, and risks, as well as the interactions among the policy options. Efforts to quantify the risks show that continued use of oral poliovirus vaccine (OPV) for routine vaccination after successful interruption of wild poliovirus transmission globally will mean continued costs and cases associated with vaccine-associated paralytic polio (VAPP) and outbreaks of circulating vaccine-derived polioviruses (cVDPVs).
Even stopping OPV will not immediately mean the immediate absence of paralytic polio, because the world faces a relatively high probability of experiencing one or more outbreaks from a cVDPV in the first five years after OPV cessation (decreasing probability for each year after OPV cessation). Although the risks of an outbreak in any individual country may seem small and it is not possible to predict where or when such future events will occur, the modelling results suggest that:
- high quality surveillance will need to continue globally
- response teams will need to be prepared to aggressively contain any outbreaks detected
- a stockpile of vaccine will be needed for outbreak response.
The modelling also suggests that cessation of OPV should be coordinated globally, and that countries should understand the increased costs and risks they might incur by stopping either earlier or later than the rest of the world.
With respect to outbreak response, a dynamic model based on the epidemiological and virological data from current and historical outbreaks identified conditions that tend to lead to larger occurrences, notably the time to detect and respond to the outbreak. Based on this modelling research in 2005, the Global Polio Eradication Initiative implemented strategies to reduce its outbreak response times. Modelling also played a key role in 2007 in helping policy makers understand the cost and case implications of abandoning the goal of eradication to switch to control.
Although models provide the opportunity to explore the potential uncertain futures and our options, they are limited by the quality of the information going into the models and by the amount of complexity that they can capture. However, modelling represents a critical tool that helps stakeholders understand the trade-offs that come with different choices in the context of an uncertain world.
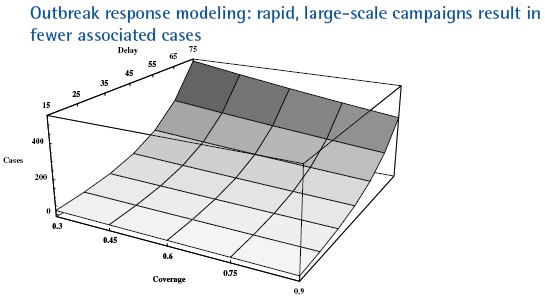
Outbreak size as a function of response delay and coverage in a low-income country with 10 million people, no routine immunization for five years after OPV cessation, acute flaccid paralysis surveillance, and low-medium R0 (=10). All response scenarios target children less than five years of age with mOPV and occur at 30-day intervals with the second and third rounds achieving 90% coverage.
In September of 2004, the Advisory Committee on Poliomyelitis Eradication (ACPE) recommended that “in order to potentially enhance the impact of SIAs by improving immune response in vaccinated children, WHO should immediately work to accelerate the process of regulatory approval of monovalent oral polio vaccine type 1 (mOPV1), with the aim of having a product available for potential use in critical endemic areas by early 2005 as an adjunct to the existing eradication activities.”
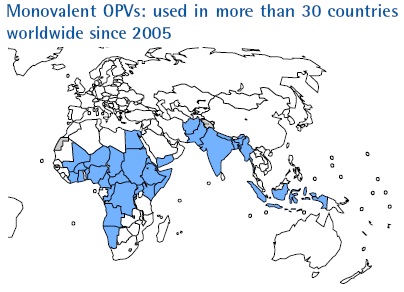
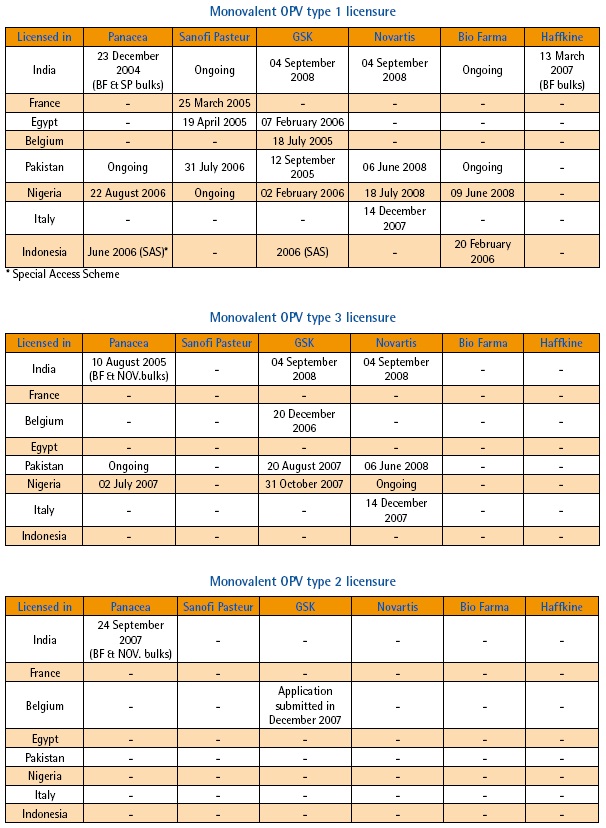
Following this recommendation, the development of mOPV1 was launched and by the following March, Sanofi Pasteur – spearheading the development process from the industry side – had a product licensed by the French regulatory authority (AFSSAPS). As of October 2008, an additional five mOPV1 products have been developed and the total number of countries with licensures is now eight. This impressive achievement has since been mirrored by the equally rapid and successful development of monovalent OPV type 3 (mOPV3). In 2007, efforts were launched to develop and license monovalent OPV type 2 (mOPV2) for eventual use in a global polio vaccine stockpile or if appropriate, for control of poliovirus type 2 outbreaks.
 |
The process from initial development through to licensing and eventually prequalification is complex and involves a number of key stages. First, there is the preparation stage which involves the assembly of all trivalent OPV (tOPV) prequalified manufacturers and related national regulatory authorities (NRAs), as was done in September 2004, immediately following the ACPE meeting. The purpose of such a meeting was to define the feasibility in terms of production and timelines, as well as the regulatory pathways. Subsequently, individual meetings were held in order to obtain indications of interest from the manufacturers and their respective NRAs. Throughout this process, UNICEF ensures a facilitated supply process by identifying the technical specifications for a mOPV tender. Furthermore, WHO pledged to purchase a minimum of 50 million doses.
WHO and the NRAs of all mOPV-producing countries agreed on an expedited approach to conduct a parallel review of the licensure dossier across both WHO and UNICEF (the traditional procurer of vaccines). This mechanism allowed licensure of mOPV in the country of origin within a few months of development. Licensure of mOPV was granted on the basis of historical clinical data generated in several countries between 1960 and 1980, however with the provision that clinical trials be conducted post-marketing to update this historical data. Part of the licensure requirements also stipulated that a clinical expert report be prepared. WHO therefore committed to commissioning such a report for each of the monovalent vaccines (since authored by Professor Peter Folb) and to implementing the necessary clinical trials, evaluating each monovalent vaccine in comparison to the trivalent version. The first of these trials was conducted in Cairo, Egypt, using Sanofi Pasteur’s mOPV1 in mid-2005. The results of this trial were recently published in the New England Journal of Medicine (1). Sanofi Pasteur’s mOPV1 was the first to achieve WHO pre-qualification.
This regulatory pathway is fully consistent with the provision made in the WHO prequalification procedure as described in the WHO document WHO/IVB/05.19 Procedure for assessing the acceptability, in principle, of vaccines for purchase by United Nations agencies. Since 2004, WHO has been facilitating the registration of mOPV through regular meetings with manufacturers and NRAs to accelerate the licensing process with respect to the specific regulations in place in each country.
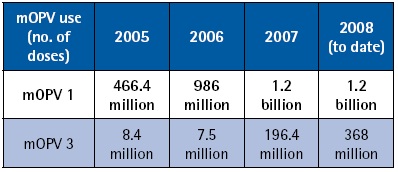 |
Most countries accept UNICEF-purchased vaccine for use. However, national policies can dictate that licensure be required in a given country to allow the use of mOPV in immunization campaigns. Such has been the case, for example, in Egypt, Pakistan, and Nigeria. Manufacturers, along with WHO staff from HQ and country offices, have been collaborating closely with NRAs to facilitate the licensure of mOPVs in order to avoid any monopoly situation and to enlarge the flexibility of vaccine supply to high risk areas. As a result, at least two mOPV1 products are licensed in Pakistan, India and Nigeria and licensure of two additional products is underway. To date, mOPV1 and mOPV3 have been used in over 30 countries. To date, nearly 4.5 billion mOPV doses have been administered since development began, testament to the successful integration of this new vaccine into the polio eradication effort, as well as to an unprecedented vaccine development effort.
Following the development and licensure of monovalent oral polio vaccine type 1 (mOPV1) in December 2004 in India, massive quantities (>2 billion doses) of this vaccine have been used in northern India (Uttar Pradesh and Bihar) for polio eradication. The public health impact of the use of this vaccine in the targeted areas has been very rewarding, with no indigenous cases of poliomyelitis caused by poliovirus type 1 detected in western Uttar Pradesh for a period >12 months, thus demonstrating the biological feasibility of eradication in this most difficult-to-eradicate area in the world. In short, endemic transmission of type 1 poliovirus was successfully interrupted from western Uttar Pradesh. However, in mid-2008, poliovirus type 1 was re-imported into some areas of western Uttar Pradesh from neighbouring Bihar. As this (ultimately successful) mOPV1 strategy was being carried out in western Uttar Pradesh, further clinical data that would demonstrate empirically the increased efficacy of this specific mOPV1 vaccine was gathered, as two clinical trials were initiated in 2005 and 2006.
In early July 2008, the trial investigators met in Vellore, India to discuss the preliminary results of the two clinical trials conducted in Indore (Madhya Pradesh) and Hyderabad (Andhra Pradesh) during the past three years. The trials evaluated regular-potency mOPV1 and higher-potency mOPV1, compared to trivalent OPV (tOPV), all formulated by Panacea Biotec Ltd. In addition, the second trial included an international comparison mOPV1 produced by Sanofi Pasteur, Lyon, France, which had been used in an initial clinical trial in Egypt in 2005 (N Engl J Med 2008, 359;16; 1655-1665). The results of the Indore and Hyderabad trials were almost identical and demonstrated that:
- a birth dose of polio vaccines (mOPV1 or tOPV) given shortly after birth results in low seroconversion rates (between 10-18% against poliovirus type 1 in Indian newborns)
- a dose of mOPV1 given at 30 days of age, in contrast, results in unexpectedly high seroconversion rates (>85% in the mOPV1 arms compared with almost 60% of trivalent OPV) against poliovirus type 1
- the higher-potency mOPV1 did not result in appreciably higher immunity gains than the regular-potency mOPV1.
The causes for the lower immunogenicity in Indian newborns are not known, but do not appear to be related to vaccine potency or biological factors, such as levels of maternally-derived antibodies or timing of feeding breast milk (colostrum). The findings of these trials confirm the superiority of OPV1 over trivalent OPV in inducing immunity against poliovirus type 1 and support the continued widespread use of this vaccine in children <5 years of age to facilitate the elimination of the last chains of poliovirus type 1 transmission in India.
The Global Polio Eradication Initiative (GPEI) continues to evaluate all vaccine options to accelerate polio eradication, including strategies of new vaccines and innovative use of existing vaccines. In November 2008, the Advisory Committee on Poliomyelitis Eradication (ACPE) recommended that the GPEI pursue a clinical evaluation of bivalent oral poliovirus vaccine (bOPV). Based on expert advice, the bOPV was formulated identical as to trivalent OPV (tOPV), except for the exclusion of Sabin type 2 poliovirus component. A clinical trial is currently underway in three sites in India, in Pune (Maharashtra), Indore (Madhya Pradesh) and Chennai (Tamil Nadu). Currently all required subjects have already been enrolled. The results from this trial are expected at the end of the first quarter of 2009. If the trial results are promising, licensure will be sought for this product, and the bOPV could then be used in areas where poliovirus types 1 and 3 are co-circulating.
As part of optimizing the use of different polio vaccines to close the immunity gaps against the three serotypes, and supported by a recommendation of the India Expert Advisory Group for Polio Eradication (IEAG) in 2008, the GPEI is planning to study to evaluate inactivated poliovirus vaccine (IPV) in late 2008 in northern India. The vaccines assessed will be fractional dose IPV (1/5th of a standard dose given intradermally), full dose IPV (administered intramuscularly) and monovalent oral poliovirus vaccine type 1 (mOPV1). If successful, fractional dose IPV could serve both as a cost-saving and supply-extending measure that could facilitate the inclusion of this vaccine for campaign use in known reservoir areas of wild-type poliovirus circulation.
In recent years, three seroprevalence surveys (i.e., assessment of antibody against the poliovirus serotypes) have provided immunity profiles of young children in Egypt, Indonesia and India. The findings of each survey have provided interesting country-specific results that have led to programmatic actions, where appropriate, and provided an immunity benchmark for the eradication initiative.
In Egypt, infants 6-11 months of age with a history of ~7 doses of trivalent oral poliovirus vaccine (tOPV), yielded high seroprevalence to type 1 and 2 (>99%), and somewhat lower levels for type 3. Elimination of transmission of wild poliovirus was achieved shortly after the survey was conducted in December 2004 (1).
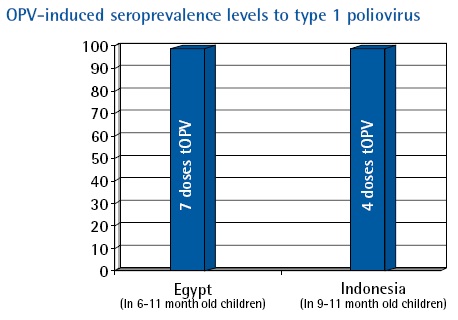 |
In Indonesia, after receipt of 4 doses of tOPV, the seroprevalence to all three poliovirus serotypes was almost 100% (2).
In Moradabad (Uttar Pradesh, India), among infants 6–12 month of age, the seroprevalence study is in the laboratory phase to determine if compromised vaccine efficacy rather than failure to vaccinate is the primary cause for the continued circulation of poliovirus in northern India. The eradication program anticipates that more seroprevalence surveys will be conducted in 2009 for programme evaluation purposes, to help assess the immunogenicity of the vaccines and to identify areas to improve programmatic action.
References
1. El-Sayed N et al. Survey of poliovirus antibodies during the final stage of polio eradication in Egypt. Vaccine, 2007, 25(27):5062–70.
2. Polio eradication: surveys of routine immunization coverage and seroprevalence against polioviruses, Yogyakarta Province, Indonesia. Weekly Epidemiological Record, 2008, 83:45–48.[pdf 282kb]








