11 October 2021
Following careful review of safety and genetic stability data from mass immunization campaigns conducted with the novel oral polio vaccine type 2 (nOPV2), the Strategic Advisory Group of Experts on immunization (SAGE) today endorsed the transition to the next use phase for the vaccine. WHO’s independent Global Advisory Committee on Vaccine Safety (GACVS) and SAGE confirmed that there were no major safety concerns associated with nOPV2 after reviewing data from campaigns that used more than 65 million doses in Nigeria, Liberia, Congo and Benin earlier this year. Rollout of nOPV2 began in March and to date approximately 100 million doses have been administered to children across seven countries.
This decision marks the end of the vaccine’s initial use period and the removal of certain use criteria for countries affected by circulating vaccine-derived poliovirus type 2 (cVDPV2) outbreaks. It means that more countries will be eligible to use the vaccine under its WHO Emergency Use Listing (EUL) recommendation, once verified for use and as supply allows.
“The move is a positive and welcome advancement as GPEI and countries strive to bring cVDPV2 outbreaks to an end, and sees the achievement of a milestone outlined in goal 2 of our strategic plan: the goal to stop cVDPV2,” commented Aidan O’Leary, WHO’s director of polio eradication. “Not only does the decision inspire further confidence in nOPV2 as a safe and effective tool, it will facilitate a smoother preparedness process for countries looking to use the vaccine in the future,” he added.
“We are very pleased with the SAGE’s endorsement of transitioning nOPV2 to the next rollout phase. Progress like this is a result of strong partnerships at every level and we must continue forging forward together, using innovative tools like nOPV2, to reach every last child and end all forms of polio for good,” said Akhil Iyer, the UNICEF Director of Polio Eradication.
Ananda Bandyopadhyay, deputy director from the Bill & Melinda Gates Foundation and co-lead of GPEI’s nOPV2 Working Group stated, “Today is an important milestone in the road to polio eradication. Innovation has always been key to progress, and this tool – the first vaccine ever to be approved under WHO’s EUL pathway – is a shining example of how the GPEI responds to challenges, but the work is far from over.” BMGF is a core partner in GPEI and has funded the development of nOPV2 from its inception.
Requirements for rollout
Prior to this transition, countries were required to meet an additional set of strict criteria to use nOPV2 during its initial use period. These were developed by GPEI and endorsed by SAGE to allow for even closer monitoring of nOPV2’s performance during its introductory phase and early large-scale uses. While countries no longer need to meet these initial use protocols, use of nOPV2 remains subject to specific post-deployment requirements under EUL, such as monitoring for safety and effectiveness.
“As we move forward into the next phase of the vaccine’s rollout, countries will still need to meet special use requirements, but they will be less onerous,” said Simona Zipursky, WHO co-lead of the GPEI’s nOPV2 Working Group. “GPEI will continue to work with all countries who wish to roll out nOPV2 to help them meet these remaining criteria,” she added.
Optimal response with available vaccine
In addition to those who have already rolled out the new tool, 16 other countries are also verified as ready to use nOPV2 by GPEI and a further 17 are in the midst of preparations. More nOPV2 campaigns are due to launch later this year, however, supply of the vaccine is limited.
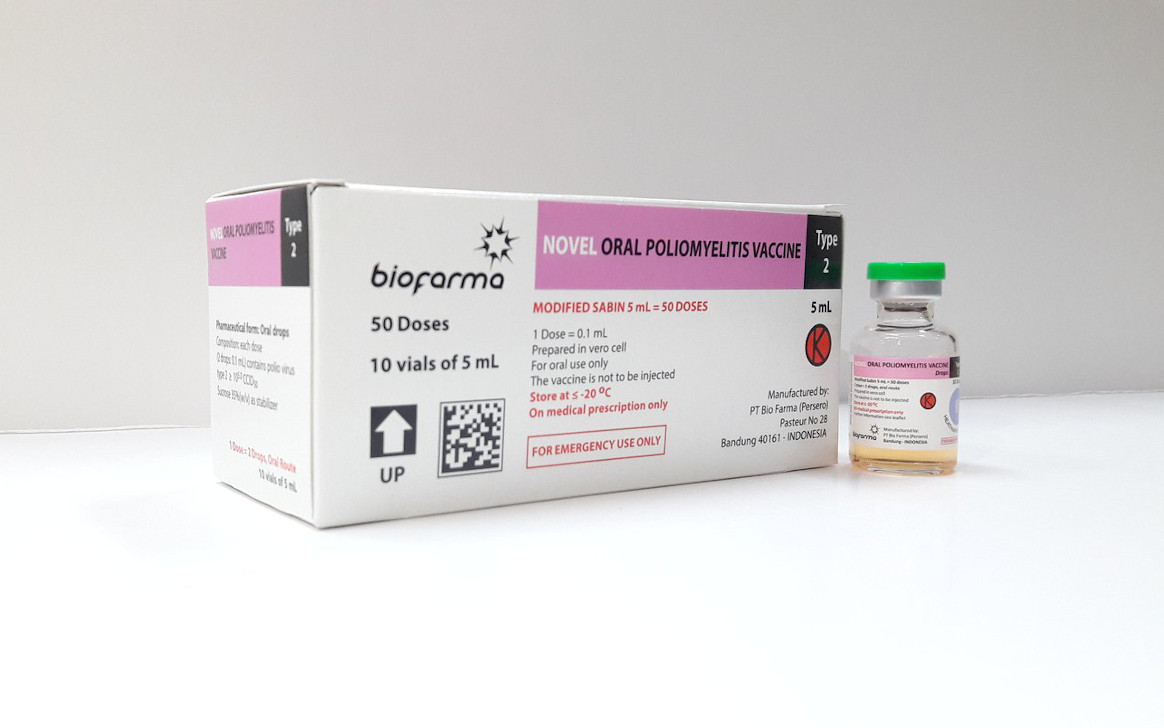
Active cVDPV2 outbreaks are ongoing in more than 20 countries across Africa and Asia and there have been recent detections of the virus in Europe. COVID-19 has impacted production of the vaccine, including by limiting supplies, available personnel and manufacturer capacity. GPEI is working with nOPV2’s manufacturer, Bio Farma, to increase supply as soon as possible and is accelerating efforts to bring a second manufacturer online. A GPEI prioritization framework will guide distribution of nOPV2 for the immediate term, until supply is increased.
“We are seeing sharp rises in demand for nOPV2, which is testament to the vaccine’s field performance, and we are working to increase supply as quickly as feasible,” said O’Leary. “We must be very clear though, that outside of nOPV2, there is no shortage of effective type-2 containing oral polio vaccines, and countries should not delay in responding to an outbreak. In line with SAGE’s guidance they should respond rapidly with whichever of the vaccines (mOPV2/nOPV2) is available to them,” he said.
With nOPV2 not yet WHO-prequalified, monovalent oral polio vaccine type 2 (mOPV2) ̶ nOPV2’s counterpart and close relative ̶ remains available to countries for outbreak response. The vaccine has a track record of successfully stopping cVDPV2 transmission and from 2019 to 2020, nearly 80% of outbreaks were closed following just two rounds of immunization. Trivalent oral polio vaccine (tOPV), containing all three vaccine serotypes, may be a more appropriate tool in situations where there is co-circulation of wild polio virus type 1 (WPV1) and cVDPV2.
“All OPVs can stop outbreaks,” said O’Leary. “Regardless of which vaccine is used, the key for any successful [outbreak] response is achieving high levels of vaccination coverage and quickly. That is what we need to remain mindful of and achieve. nOPV2 is only one of the effective vaccines in our toolkit and GPEI will continue to support countries to respond as rapidly as possible to outbreaks, as per the SAGE guidance.”
Next steps for nOPV2 development
Polio remains a Public Health Emergency of International Concern (PHEIC) under International Health Regulations, enabling nOPV2’s continued use through EUL. Field data collection and analyses will be ongoing to support the vaccine’s prequalification and full licensing, expected in 2023. Among other studies, a phase III clinical trial is currently underway in the Gambia.