When we consider eradication of a disease, we might think that this means total, 100% absence of the virus that causes it from the globe. It’s a fair assumption. But for polio, while this is expected to be the case in terms of viral presence in humans (through infection), and detection in the environment, poliovirus is and will continue to be handled and stored in places it is still needed to ensure public safety, such as vaccine manufacturing facilities and laboratories. Polio vaccines will be required for years to come, and key research and diagnostic work will need to be sustained – both activities which call for ongoing use of the virus.
Working with eradicated pathogens, however, is a risk but one that can be minimized with appropriate safeguards. To date, two of three strains of wild poliovirus have been wiped out globally and WHO is working with its Member States to reduce the number of places holding these viruses to a minimum, and to destroy unneeded stocks. For those opting to keep polioviruses to carry out critical functions as mentioned above, strict biorisk management ̶ or ‘containment’ – against WHO standards is vital to help make sure they don’t escape facilities to once again cause outbreaks in communities, and the world at large.
As one of 25 countries retaining the eradicated type 2 poliovirus strain, Canada is putting in place the necessary safeguards to minimize this risk. In fact, the country is the first to have one of its facilities move to the second stage of WHO’s Containment Certification Scheme, through receiving an ‘Interim Certificate of Containment’, or ICC.
“There are three stages of containment certification and ICC is the second,” said Liliane Boualam, WHO containment technical officer and co-chair of the GPEI’s Containment Management Group. “Effectively, this means the facility in question has been audited against WHO containment guidance by its National Authority for Containment, and has met a certain threshold of containment requirements. The validity of the ICC is limited to three years and the facility has two options: to either address any non-conformities identified through the audit, so as to be compliant with WHO containment guidance (GAP), or to decommission the facility and destroy or transfer virus materials to another (facility) undergoing certification within those three years,” she added. “WHO and GPEI commend Canada and its facility for being the first to achieve ICC status, and we look forward to their next steps, and progress by other countries retaining (polio)virus,” she said.
“We are very pleased to have this Canadian facility advance to the next step in the containment certification process,” said Andréanne Bonhomme, Director, Biosafety and Biocontainment Operations at the Public Health Agency of Canada, and National Authority for Containment chair. “Canada appreciates the responsibility that comes with the ongoing handling and storage of poliovirus, and is committed to ensuring safe and secure containment of these viruses as essential functions continue,” she added.
Global progress, but many countries behind schedule
Containment of type 2 poliovirus came into effect in 2016, following the declaration of its eradication in 2015. In 2018, WHO Member States recognizing the importance of the work, committed to acceleration of containment action globally. Significant advances have been made however many countries are behind on implementation timelines*. In part, the pandemic complicated matters, diverting resources away from containment and slowing implementation.
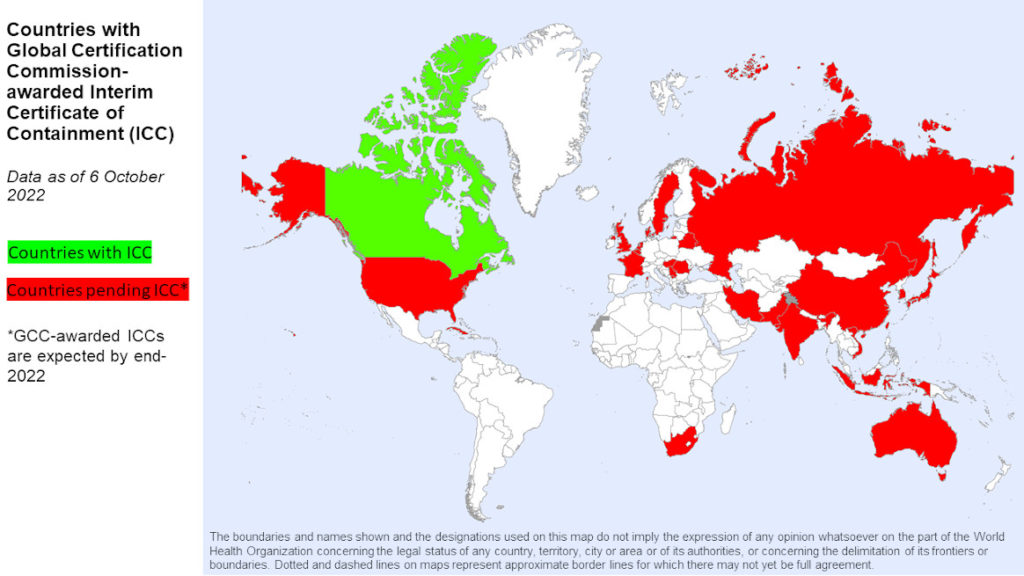
“COVID-19 certainly hindered poliovirus containment efforts,” said Professor David Salisbury, chair of the Global Commission for the Certification of Eradication of Poliomyelitis and final signatory for containment certification certificates. “Encouragingly, we have seen improvements since individual country situations have stabilized, but progress is considerably behind schedule and we need to reprioritize this important work,” he added. “The goal is to have all facilities retaining polioviruses to have achieved full containment certification by 2026 and this means a great amount of work, and indeed much catching-up, needs to take place now,” he added.
Revised guidance and new strategy
In an effort to streamline containment guidance and boost implementation by countries, WHO revised its Global Action Plan for Poliovirus Containment (GAPIV) in July 2022, following an in-depth consultation process with country stakeholders. A new containment-dedicated strategy and action plan has also been developed by the GPEI to aid progress.
“The strategy and action plan elaborate on containment principles included in GPEI’s overall eradication strategy, and provide distinct objectives, milestones, timelines and ways forward for countries for containment implementation. We are confident that together with the revised GAPIV guidance, these tools will help countries make bigger and stronger strides forwards,” said Boualam.
“Canada welcomes and appreciates the revised guidance, for which it provided significant risk based input along with other WHO Member States,” said Bonhomme. “Containment is a global responsibility and Canada will continue to play its part towards the global eradication, with WHO’s support,” she said.
There are currently 65 facilities worldwide officially designated by national governments for the retention of type 2 poliovirus. All facilities are urged to ensure applications for their ICCs by the end of 2022.