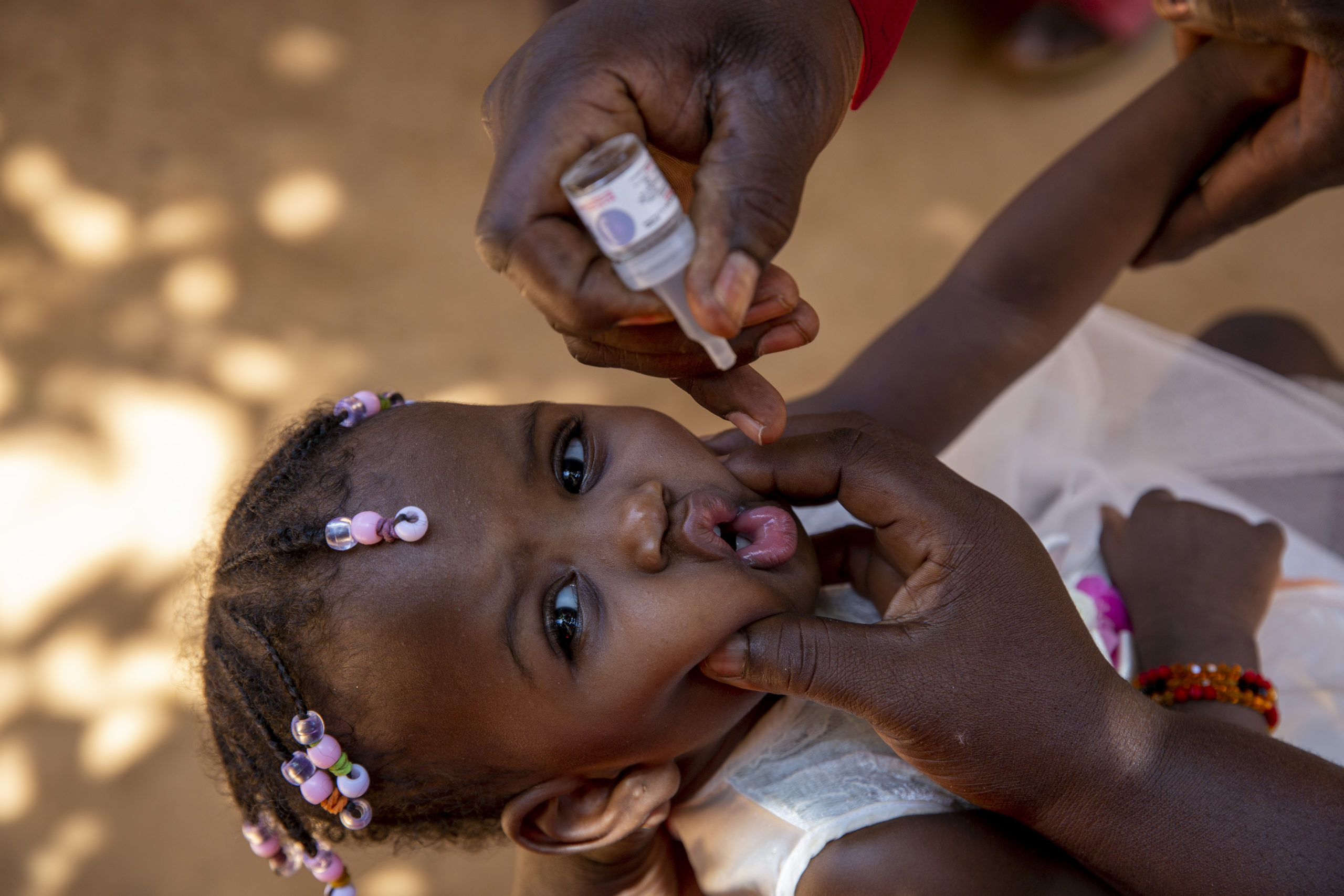
In late December 2023, the World Health Organization issued its first-ever prequalification approval for a vaccine being used under its Emergency Use Listing (EUL) regulatory pathway – novel oral polio vaccine type 2 (nOPV2). Since rollout of this next-generation vaccine began in March 2021, the Global Polio Eradication Initiative (GPEI) has administered nearly 1 billion doses of nOPV2 across 35 countries, protecting millions of children against illness and paralysis. Prequalification will enable additional countries to access the vaccine more easily for more sustainable response to outbreaks of type 2 variant poliovirus (cVDPV2).
“This is a historic milestone for polio eradication and for public health,” said WHO Director-General Dr Tedros Adhanom Ghebreyesus. “Novel oral polio vaccine type 2 has blazed a trail for other new vaccines that address critical health emergencies, and its use demonstrates the utility of the EUL mechanism in helping to rapidly get new products to where they’re needed most.”
The EUL to PQ pathway
WHO EUL is reserved for the use of yet-to-be-licensed vaccines, medicines and diagnostic tools during public health emergencies like polio outbreaks. Following rigorous assessments of existing quality, safety and efficacy data from completed clinical trials, the pathway enables expedited availability of products to the places impacted by these emergencies. The vaccine’s manufacturer, Bio Farma Indonesia, has been instrumental in ensuring supply and enabling nOPV2 to earn full licensure from the Indonesian regulatory authority, Badan POM. WHO Prequalification (PQ) is the final step of the process, allowing for streamlined regulatory approval for nOPV2 use in countries that need it.
“This key step illustrates how innovation can help protect children against the variant poliovirus type 2, with thanks to the support of donors and partners, and the commitment of governments and community health workers,” said UNICEF Executive Director Catherine Russell. “UNICEF is committed to helping ensure the safe and adequate supply of vaccines to countries, while working with communities to build trust in vaccines. We need to keep going till we reach every child, and eradicate polio once and for all.”
nOPV2’s performance and the power of innovation
To date, nOPV2 has been used in 35 countries under EUL, predominantly in the African region which is most affected by cVDPV2 outbreaks. Throughout its clinical development and field use, nOPV2 has proven to be as safe to use and effective at stopping outbreaks as its predecessor, monovalent type 2 oral vaccine (mOPV2), but, importantly, is more genetically stable. After nearly three years of use, estimates show that nOPV2 is 80% less likely to seed new variant polio outbreaks, making it the tool of choice to stop these outbreaks for good.
Nigeria has played an outsized role in nOPV2 rollout in the leadup to WHO Prequalification, administering nearly half a billion doses to children across the country to date. The vaccine has helped bring about an 85% reduction in variant poliovirus cases in Nigeria since 2021, and its impact is visible through this and other data in a new story from the GPEI.
Development of the vaccine began in 2011 through a consortium of experts led by the Bill & Melinda Gates Foundation, including the UK National Institute for Biological Standards and Controls (NIBSC), the U.S. Centers for Disease Control and Prevention (US-CDC), the US Food and Drug Administration, PATH and the University of California at San Francisco.
“Supporting the development of new vaccines is one of the most important investments we can make to protect people against preventable diseases like polio,” said Mark Suzman, CEO of the Bill & Melinda Gates Foundation. “With easier access to nOPV2 for more countries, this vaccine will keep even more children safe in areas still grappling with poliovirus. We look forward to working with partners across sectors to support more groundbreaking innovations.”
Next steps in the fight against type 2 variant poliovirus
As of 3 January 2024, 325 cases of cVDPV2 had been reported in 2023, compared to 689 cases in 2022. While nOPV2 has played a key part in this reduction, its success, like any polio vaccine, depends on the ability to rapidly implement high-quality immunization campaigns that reach every child.
To overcome the final challenges that remain in polio eradication, the GPEI is finding new ways to access children living in hard-to-reach areas, promote community acceptance of vaccines, and improve early detection and response to outbreaks. These efforts are being prioritized in the places where children are at the highest risk of encountering and spreading the virus.
“It is critical to protect all children against polio with timely administration of vaccines. Along with our global partners, CDC is committed to ensuring rapid detection of type 2 polio outbreaks and response with the novel oral vaccine,” said Dr. Mandy Cohen, Director of the US-CDC.
A prequalified nOPV2 will help to make important headway against cVDPV2 outbreaks, and with renewed support from global partners, donors and leaders of polio-affected countries to fully implement the program’s strategy, we can stop all forms of polio for good.
END
—
About the GPEI:
The Global Polio Eradication Initiative is a public-private partnership led by national governments with six partners – the World Health Organization (WHO), Rotary International, the US Centers for Disease Control and Prevention (CDC), the United Nations Children’s Fund (UNICEF), Bill & Melinda Gates Foundation and Gavi, the vaccine alliance. Its goal is to eradicate polio worldwide.
For media enquiries:
WHO
For prequalification specific enquiries:
Sarah Sheppard – Communications Officer sheppards@who.int
For polio and nOPV2 enquiries:
Joseph Swan – Communications Officer swanj@who.int
UNICEF
Helen Wylie, Communications Specialist hwylie@unicef.org
Bill & Melinda Gates Foundation
Amber Zeddies, Senior Program Officer amber.zeddies@gatesfoundation.org
US CDC
Chelsea Toledo, Health Communications Specialist rnv8@cdc.gov