20 September, Bali – In an important step towards a polio-free world, the Global Commission for the Certification of Poliomyelitis Eradication (GCC) today concluded that wild poliovirus type 2 (WPV2) has been eradicated worldwide. The GCC reached its conclusion after reviewing formal documentation submitted by Member States, global poliovirus laboratory network and surveillance systems. The last detected WPV2 dates to 1999, from Aligarh, northern India.
20 September, Bali – In an important step towards a polio-free world, the Global Commission for the Certification of Poliomyelitis Eradication (GCC) today concluded that wild poliovirus type 2 (WPV2) has been eradicated worldwide. The GCC reached its conclusion after reviewing formal documentation submitted by Member States, global poliovirus laboratory network and surveillance systems. The last detected WPV2 dates to 1999, from Aligarh, northern India.
This announcement marks a major landmark in the global efforts to eradicate all three wild poliovirus serotypes: WPV1, WPV2 and WPV3. WPV3 has not been detected globally since November 2012 (in Nigeria); the only remaining endemic WPV1 strains are now restricted to Pakistan and Afghanistan.
The timing of this declaration of WPV2 eradication is also a significant step in preparation for the phased removal of oral polio vaccines (OPVs), beginning with the removal of type 2 oral polio vaccine requiring a switch from using trivalent OPV (containing all three serotypes) to bivalent OPV (containing only type 1 and 3 serotypes, but not type 2) in OPV-using countries, planned for April 2016.
Meeting one prerequisite for the switch.
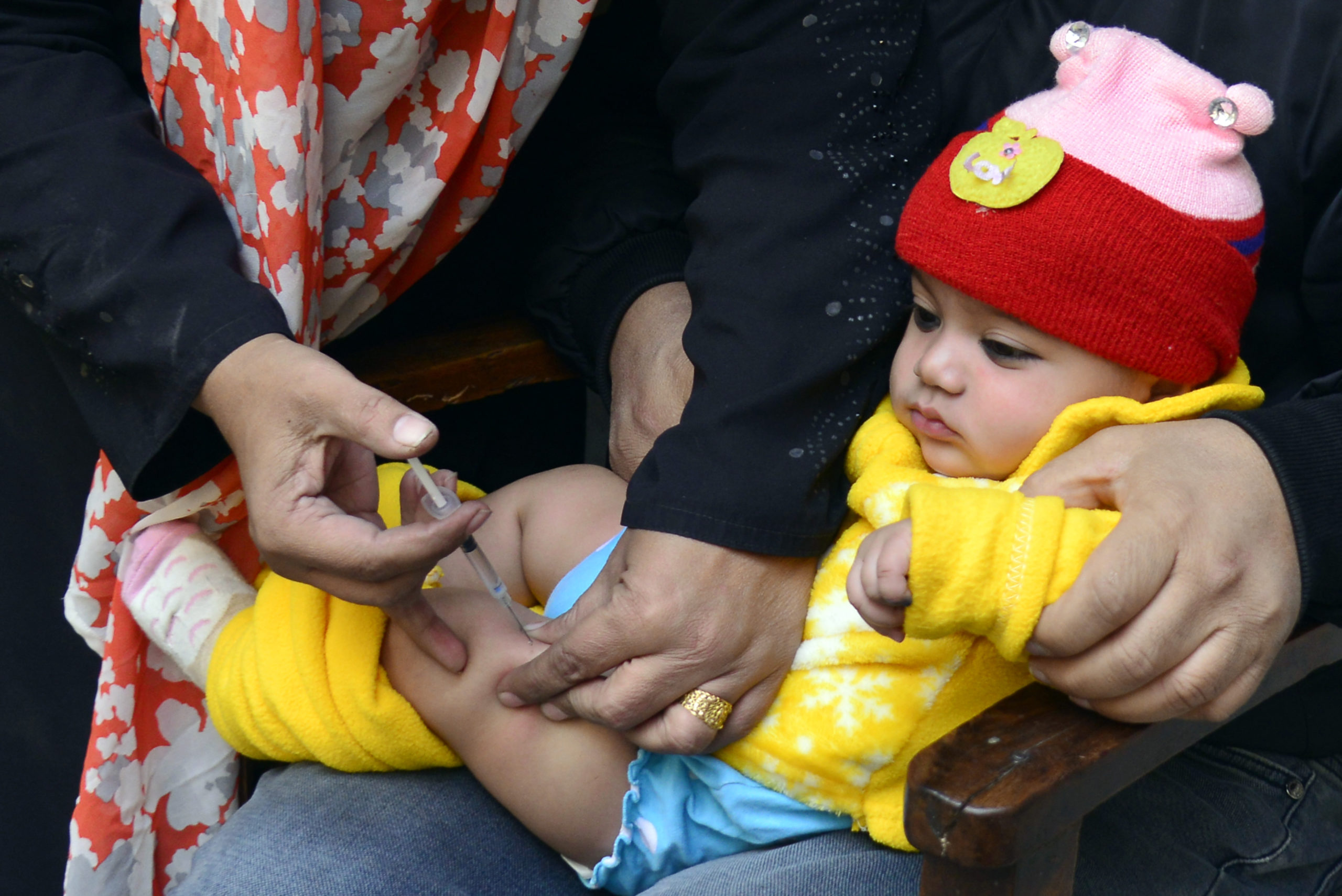
OPV contains attenuated (weakened) polioviruses. On extremely rare occasions, use of OPV can result in cases of polio due to vaccine-associated paralytic polio (VAPP) and circulating vaccine-derived polioviruses (cVDPVs). For this reason, the global eradication of polio requires the eventual cessation of all OPV. With WPV2 transmission already having been successfully interrupted, the only type 2 poliovirus which still, on very rare occasions, causes paralysis is the type 2 serotype component in trivalent OPV. The continues use of this vaccine component is therefore inconsistent with the goal of eliminating all paralytic polio disease.
The continued occurrence of polio cases caused by type 2 vaccine-derived poliovirus is the reason to implement the switch from trivalent OPV to bivalent OPV in routine immunization programmes, even before the remaining strains of wild poliovirus are eradicated. Following WPV1 and WPV3 eradication, use of all OPV in routine immunizations will subsequently be stopped. Recent new cVDPV outbreaks in Ukraine (type 1 cVDPV) and the Guinea/Mali border area (type 2 cVDPV) this year further underscore the need for the phased removal of OPVs beginning with type 2 next year.
The switch is expected to be associated with significant public health benefits. More than 90% of all cVDPV outbreaks are caused by the type 2 component of trivalent OPV. Also, up to 38% of all VAPP cases are estimated to be caused by this component. The disease burden due to type 2 vaccine viruses is expected to drop to zero after the planned switch is implemented globally.
To prepare for the switch in April 2016, a number of criteria must be met, as guided by the Strategic Advisory Group of Experts on immunization (SAGE), the independent expert body advising the World Health Organization (WHO) on all matters relating to immunization. These criteria include:
- introducing inactivated polio vaccine (IPV) in all routine immunization programmes to maintain immunity levels to type 2 polio (IPV is a trivalent vaccine manufactured from all three inactivated/killed vaccine strains, which are not associated with vaccine-associated paralysis);
- securing access to bivalent OPV licensed for use in routine immunization in OPV-using countries (bivalent is currently licensed primarily for supplementary immunization activities);
- ensuring global outbreak response capacity, including through a global stockpile of monovalent OPV type 2 (mOPV2), to rapidly enable a response should any vaccine-derived type 2 poliovirus still emerge following the switch;
- securing all WPV2 and Sabin type 2 viruses remaining in a small number of essential facilities under appropriate biocontainment levels to minimise the risk of re-introduction into a type 2 polio-free world; and,
- declaration by the independent Global Commission that WPV2 has been eradicated globally.
This week’s declaration has satisfied the fifth criteria outlined above, removing a major hurdle and further paving the way for the trivalent OPV to bivalent OPV switch next year, as planned. SAGE will convene at end-October in Geneva, Switzerland, to further review countries’ preparatory plans for next year’s switch and offer additional guidance as needed. In the meantime, a major step towards securing a lasting world free of all polio paralysis, has been taken.