Why OPV cessation?
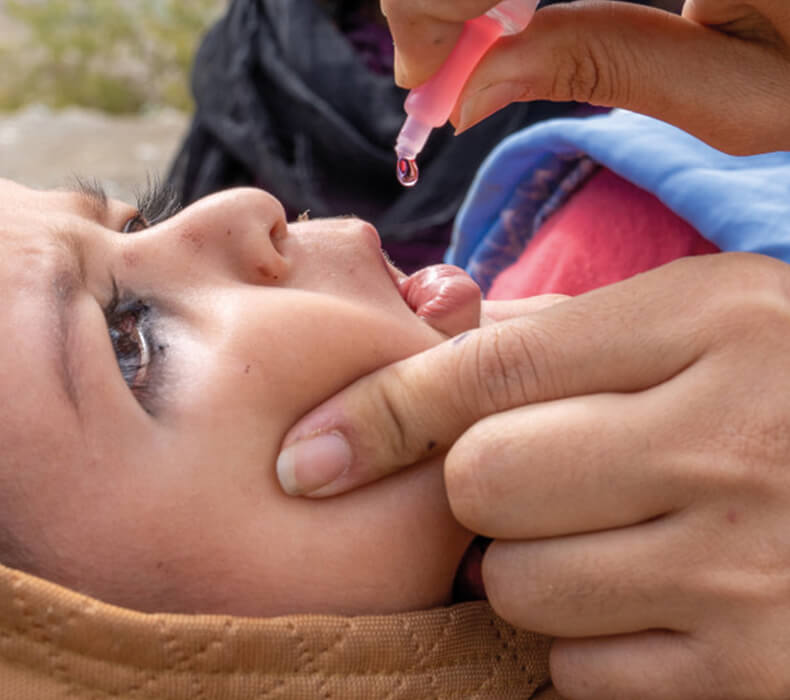
Oral polio vaccine (OPV) is extremely safe and effective at protecting children against lifelong polio paralysis. Over the past ten years, more than 10 billion doses of OPV have been given to nearly three billion children worldwide. More than 16 million cases of polio have been prevented, and the disease has been reduced by more than 99%. It is the appropriate vaccine through which to achieve global polio eradication.
OPV contains attenuated (weakened) polioviruses. On extremely rare occasions, use of OPV can result in cases of polio due to vaccine-associated paralytic polio (VAPP) and circulating vaccine-derived polioviruses (cVDPVs). For this reason, the global eradication of polio requires the cessation of all OPV in routine immunization, as soon as possible after the eradication of wild poliovirus (WPV) transmission.
Phased approach to OPV cessation – trivalent OPV to bivalent OPV switch
Historically, OPV has been available in different formulations:
- Trivalent OPV – containing type 1, 2 and 3 serotypes
- Bivalent OPV – containing type 1 and 3 serotypes
- Monovalent OPV – containing one serotype (ie type 1, 2 or 3)
A mix of all three formulations was being used to eradicate polio during supplementary immunization activities (SIAs). However, only trivalent OPV was used in routine immunization programmes. Bivalent OPV was exclusively used during SIAs to more rapidly interrupt the transmission of WPV1 and 3 – the only remaining WPV strains in circulation. WPV2 has been eradicated since 1999.
With the transmission of WPV2 already successfully interrupted, the only cases of type 2 paralytic polio were being caused by the type 2 serotype component in trivalent OPV. Over 90% of cVDPV cases were due to the type 2 component, which was also responsible for up to 38% of VAPP cases (approx. 200 cases per year worldwide).
The switch
In April 2016 a switch was implemented from trivalent OPV to bivalent OPV in routine immunization programmes.
Following WPV1 and WPV3 eradication, use of all OPV in routine immunizations will be stopped.