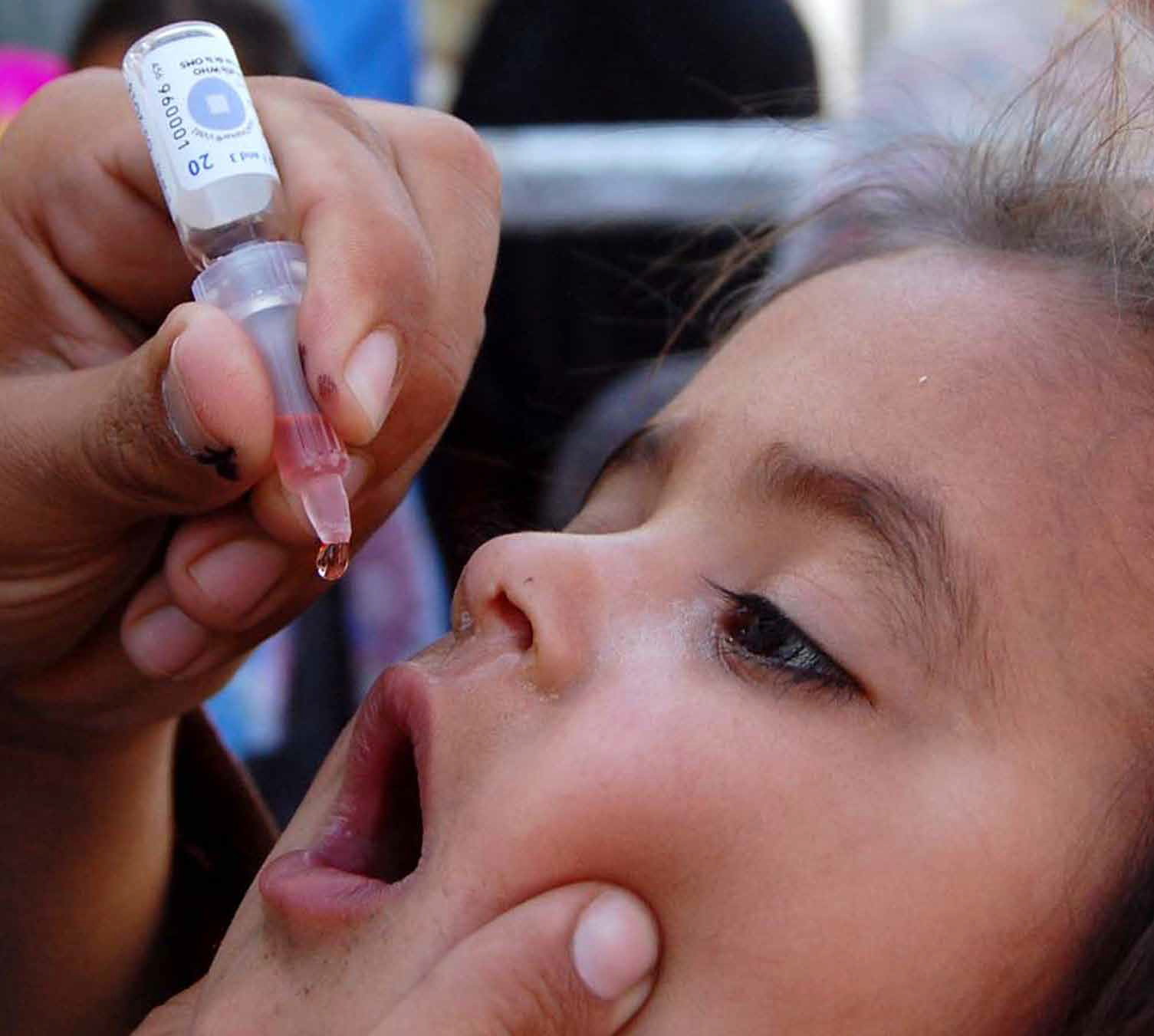
Certification of polio eradication is conducted on a regional basis. Each region can consider certification only when all countries in the area demonstrate the absence of wild poliovirus transmission for at least three consecutive years in the presence of certification standard surveillance.
In addition, all facilities holding wild poliovirus infectious and potentially infectious materials must have implemented bio-containment measures according to the Global action plan for laboratory containment of wild poliovirus (2003).
Status
Region |
Year certified wild polio-free |
| WHO African Region | 2020 |
| WHO Region of the Americas | 1994 |
| WHO South-East Asia Region | 2014 |
| WHO European Region | 2002 |
| WHO Western Pacific Region | 2000 |