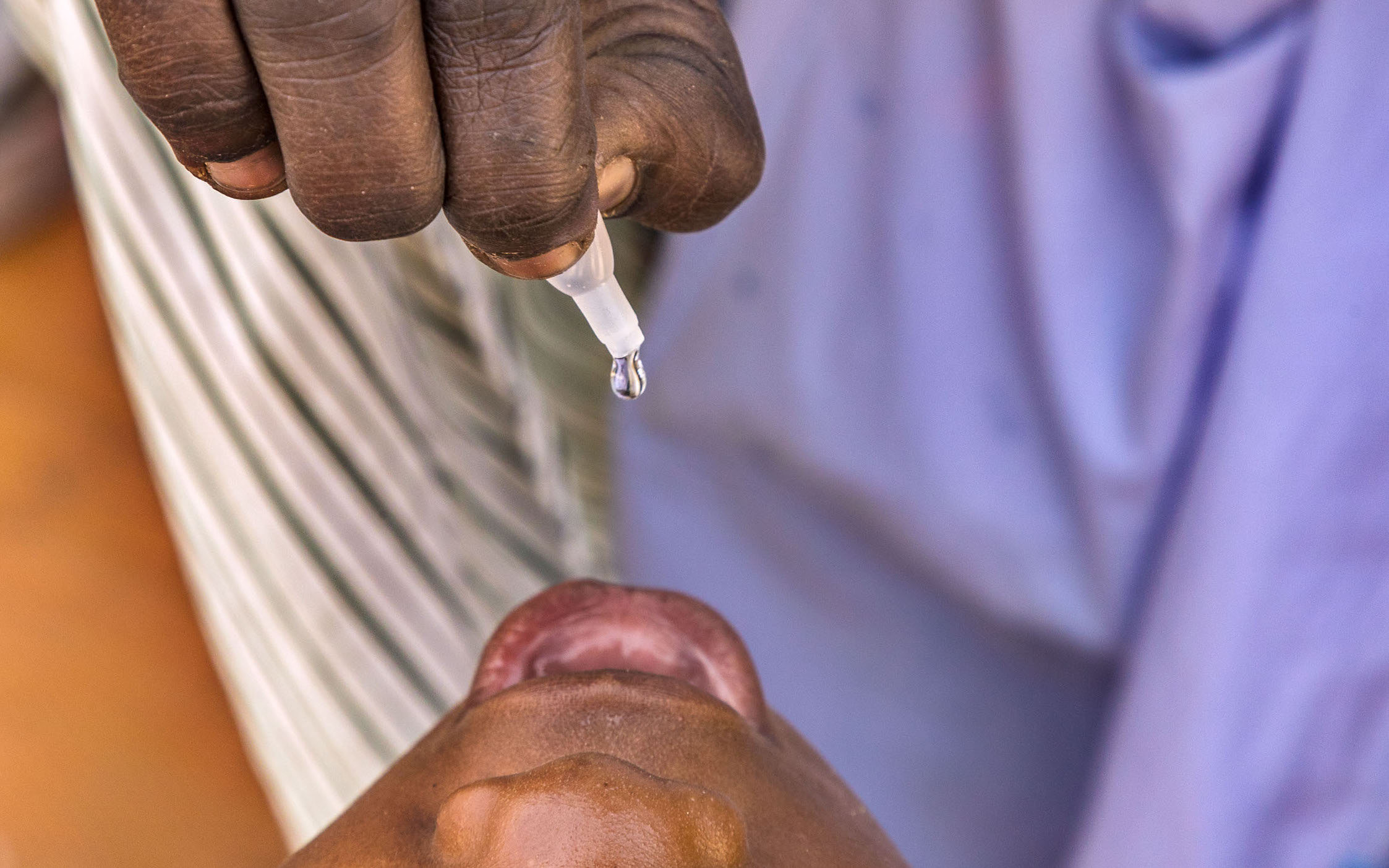
As the GPEI moves closer to eradication, the original vaccine against polio – the inactivated polio vaccine (IPV) – is playing a central role in immunizing children against polioviruses. By finding innovative methods of delivering the vaccine in a fractional dose, the GPEI is exploring new ways to overcome global supply constraints, and could help to bring the benefits of IPV to children everywhere.
The role of IPV in the Polio Endgame
Thanks to major steps forward including the declaration of wild poliovirus type 2 as eradicated in September 2015, the phased removal of oral polio vaccines began in April 2016 with the withdrawal of the type 2 component. As an essential part of this, all countries agreed to introduce one dose of IPV into their routine immunization systems to boost immunity against poliovirus types 1 and 3 and provide a baseline of immunity against type 2 in case of an outbreak of type 2 vaccine derived polioviruses.
Despite the importance of this step towards the Endgame, there are challenges facing IPV introduction. Shortfalls in IPV supply globally have left more than 40 countries without sufficient IPV supply to vaccinate all children through their routine immunization systems.
Fractional dose IPV is an innovation that could help address this challenge.
Fractional dose IPV
Full dose IPV is delivered through an intramuscular injection. But IPV can also be given intradermally, into the skin. When delivered in this way, only 1/5 of a dose is needed to generate almost as much immunity as one full dose delivered into the muscle; and two fractional doses generates higher immunity than one full dose. This innovation is making financial savings by reducing the cost of IPV, and enabling the limited supply to go much further.
 Rolling out fractional dose IPV
Rolling out fractional dose IPV
The feasibility of using this new approach to delivering IPV is supported by a growing body of research. This has led to the recommendation of the Strategic Advisory Group of Experts on immunization (SAGE) for countries to consider adopting fractional dose IPV in both their supplementary and routine immunization activities.
A new field study in Sri Lanka provided evidence that using fractional dose IPV is as effective as using a full dose in OPV primed populations to boost mucosal immunity. This joins a body of previous studies that had already shown it to be as effective as full dose at inferring humoral immunity. Mucosal immunity is critical in stopping the person-to-person spread of virus.
Further research is showing that this approach to IPV delivery can be used for both routine immunization and outbreak response, meaning that the supply that is available can be maximised. A recent pilot campaign in Pakistan gathered data on its use in campaigns, which led to the GPEI recommending its use in outbreak response to boost immunity alongside OPV. A few countries are already adopting this new approach. Recent campaigns in India and Pakistan gathered data on its use in campaigns, which demonstrated feasibility in outbreak response to boost immunity alongside OPV. In addition, India and Sri Lanka are beginning to use fractional dose IPV in their routine immunization schedules. More countries are to follow in the face of the global IPV shortage.
The GPEI is also continuing to explore additional delivery mechanisms to overcome potential operational challenges, such as adaptors and needle-free devices to make it easier to deliver the vaccine intradermally. With the progress being made in this arena, the benefits fractional dose IPV could provide for children far outweigh any challenges.
| The Global Polio Eradication Initiative (GPEI) is highlighting the innovations that are helping to bring us closer to a polio-free world. Find out about other new approaches driving the polio eradication efforts by reading more in the Innovation Series. |